Tirzepatide: A Detailed Overview for Florida, USA
Tirzepatide is a novel, injectable medication that has gained significant attention in the United States, including Florida, for its effectiveness in managing type 2 diabetes and promoting substantial weight loss. It is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. This unique mechanism of action targets multiple pathways involved in glucose regulation and appetite control.
Here’s a comprehensive breakdown of Tirzepatide:
- What it is:
Tirzepatide is a synthetic peptide that mimics the actions of two natural incretin hormones in the body: GIP and GLP-1.
Incretins are hormones released from the gut in response to food intake. They play a crucial role in regulating blood sugar levels and appetite.
By activating both GIP and GLP-1 receptors, Tirzepatide exerts a more comprehensive effect on glucose homeostasis and weight management compared to medications that target only one of these pathways.
- How it Works:
Tirzepatide’s dual action leads to several beneficial effects:
GLP-1 Receptor Agonism:
Increased Insulin Secretion: Stimulates the pancreas to release insulin in a glucose-dependent manner (primarily when blood sugar is high), helping to lower blood glucose levels.
Decreased Glucagon Secretion: Suppresses the release of glucagon (a hormone that raises blood sugar) when glucose levels are already elevated.
Slowed Gastric Emptying: Reduces the rate at which food leaves the stomach, promoting a feeling of fullness and potentially reducing overall food intake.
Reduced Appetite and Increased Satiety: Acts on the brain to decrease hunger signals and increase feelings of fullness.
GIP Receptor Agonism:
Enhanced Insulin Secretion: May also stimulate insulin release, although potentially with less glucose dependency than GLP-1.
Improved Insulin Sensitivity: May help the body’s cells become more responsive to insulin.
Potential Effects on Fat Metabolism and Satiety: Research suggests GIP may play a role in these areas, contributing to the overall weight loss effect.
- Approved Uses in the USA (Including Florida):
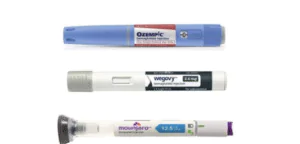
Tirzepatide is approved by the Food and Drug Administration (FDA) under two brand names:
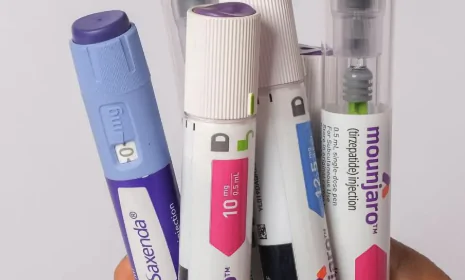
Mounjaro: Primarily indicated for the treatment of type 2 diabetes in adults, alongside diet and exercise. It can be used alone or in combination with other diabetes medications.
Zepbound: Specifically indicated for chronic weight management in adults with obesity (BMI ≥ 30 kg/m²) or overweight (BMI ≥ 27 kg/m²) who also have at least one weight-related condition (e.g., high blood pressure, high cholesterol, type 2 diabetes, sleep apnea). It is used in conjunction with a reduced-calorie diet and increased physical activity.
- Efficacy for Weight Loss:
Clinical trials (SURMOUNT program) have demonstrated significant and dose-dependent weight loss in individuals taking Tirzepatide. Average weight loss in the highest approved dose group reached 15-20% or more of baseline body weight over about 18 months, significantly greater than placebo.
A high percentage of participants achieved clinically meaningful weight loss thresholds (e.g., 5%, 10%, 15% or more).
Studies have also shown Tirzepatide to be more effective for weight loss than some other GLP-1 receptor agonists.
- Efficacy for Type 2 Diabetes:
Clinical trials (SURPASS program) showed that Tirzepatide significantly improved HbA1c levels and fasting glucose compared to placebo and other diabetes medications, including other GLP-1 receptor agonists and insulin.
Many participants achieved target glycemic goals, and some experienced weight loss as an additional benefit.
- Dosage and Administration:
Tirzepatide is administered as a once-weekly subcutaneous injection.
The starting dose is typically low (e.g., 2.5 mg) and gradually increased over several weeks (titration) to the maintenance dose (5 mg, 10 mg, or 15 mg) as determined by a healthcare provider based on individual response and tolerability.
The injection site can be the thigh, abdomen, or upper arm.
- Potential Side Effects:
Like all medications, Tirzepatide can have side effects. The most common are usually mild to moderate and gastrointestinal in nature:
Nausea
Vomiting
Diarrhea
Constipation
Abdominal pain
Decreased appetite
Less common but potentially more serious side effects can include:
Pancreatitis
Gallbladder problems
Hypoglycemia (low blood sugar), especially when used with other medications that lower blood sugar.
Allergic reactions
Individuals in Florida should discuss potential side effects and any concerns with their healthcare provider.
- Important Considerations for Individuals in Florida:
Prescription Only: Tirzepatide requires a prescription from a licensed healthcare provider.
Not a Substitute for Lifestyle Modifications: It is most effective when used in conjunction with a healthy diet and regular physical activity.
Individualized Treatment: Dosage and treatment plans will be tailored to the individual’s specific needs and medical history.
Monitoring: Regular monitoring by a healthcare provider is necessary to assess efficacy and manage any side effects.
Insurance Coverage: Coverage for Tirzepatide can vary depending on insurance plans in Florida and across the US. Prior authorization may be required.
- Who Should Not Take Tirzepatide?
Tirzepatide is not suitable for everyone. Contraindications and precautions include:
Allergy to tirzepatide or any of its ingredients.
Personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2 (for Mounjaro, the label carries a boxed warning for this).
Pregnancy and breastfeeding.
Severe gastrointestinal disease.
Your healthcare provider will assess your medical history to determine if Tirzepatide is safe for you.
- Long-Term Use and Ongoing Research:
Long-term studies are ongoing to further evaluate the safety and efficacy of Tirzepatide for both diabetes and weight management, including its impact on cardiovascular outcomes and other long-term health parameters.
In Conclusion for Florida:
Tirzepatide represents a significant advancement in the management of both type 2 diabetes and obesity. Its dual mechanism of action leads to substantial weight loss and improved blood sugar control, along with potential benefits for other cardiometabolic risk factors. However, it is crucial to use Tirzepatide under the guidance of a healthcare provider, understand its potential side effects, and recognize that it is most effective when combined with lifestyle modifications. As a resident of Florida considering or using Tirzepatide, open communication with your medical team is essential for safe and successful treatment.
Tirzepatide: A Detailed Overview for Florida, USA
Route
Doctor G Medical Excellence: Health Well-being and Longevity
URL: https://doctorgmed.com/
| Monday | 09:00 - 17:00 |
| Tuesday | 09:00 - 17:00 |
| Wednesday | 09:00 - 17:00 |
| Thursday | 09:00 - 17:00 |
| Friday | 09:00 - 17:00 |
| Saturday | 09:00 - 17:00 |
| Sunday | Closed |