Say Goodbye to Excess Weight with Tirzepatide
“Say Goodbye to Excess Weight with Tirzepatide,” framed specifically for the context within the USA as of April 10, 2025.
While the phrase is catchy and reflects the significant hope and impressive results associated with this medication, it’s essential to understand the full picture—the science, the process, the benefits, the risks, and the realities of its use in the American healthcare landscape.

- What is Tirzepatide (The Tool to Help “Say Goodbye”)?
Medication & Brands: Tirzepatide is the active ingredient. In the USA, it’s available under two FDA-approved brand names:
Zepbound®: Specifically approved by the FDA for chronic weight management.
Mounjaro®: FDA-approved to improve blood sugar in adults with Type 2 Diabetes (it also causes significant weight loss).
Mechanism: It’s a first-in-class dual GIP and GLP-1 receptor agonist. This means it mimics two key natural gut hormones that regulate appetite, food intake, and metabolic processes.
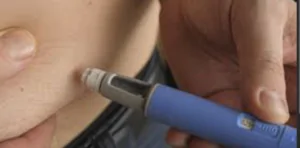
Administration: It’s a once-weekly injectable medication that patients typically self-administer using a pre-filled pen device.
- Why the Excitement? The Potential to “Say Goodbye” to Weight Struggles:
The enthusiasm and “breakthrough” language surrounding Tirzepatide stem primarily from its unprecedented effectiveness shown in clinical trials reviewed by the FDA:
Magnitude of Weight Loss: In the key SURMOUNT-1 trial for Zepbound (in people without diabetes), participants taking the highest dose (15 mg) lost an average of ~20.9% of their starting body weight over 72 weeks. This level of efficacy from a medication was previously largely unseen and begins to approach results observed with some types of bariatric surgery. Substantial weight loss was also seen at lower doses.
Targeting Biology: Unlike simply dieting, Tirzepatide works by targeting the body’s complex hormonal signaling systems that regulate hunger, fullness (satiety), and energy balance. Many users report a significant reduction in “food noise” (constant thoughts about food) and cravings, making it biologically easier to consume fewer calories without feeling constantly deprived. This addresses the physiological factors that often make sustained weight loss incredibly difficult.
High Responder Rate: A very large percentage of participants in the trials achieved clinically significant weight loss (e.g., ≥5%, ≥10%, ≥15%, ≥20%), suggesting its effectiveness across a broad range of individuals who meet the criteria.
For many people in the US who have struggled for years with excess weight despite trying various diets and exercise programs, Tirzepatide offers a powerful biological assist that makes meaningful weight loss feel achievable, hence the sentiment of potentially “saying goodbye” to that long-standing struggle.
- How Tirzepatide Facilitates Weight Loss:
Reduces Appetite & Increases Satiety: By activating GIP and GLP-1 receptors in the brain.
Slows Gastric Emptying: Helps you feel fuller for longer after meals.
Improves Insulin Sensitivity: Helps the body use glucose more effectively.
- The Reality of Using Tirzepatide (It’s a Process, Not Magic):
Prescription Medication: It requires a prescription from a licensed US healthcare provider (Doctor, NP, PA) after a medical evaluation to ensure it’s appropriate and safe for you.
Lifestyle Integration: It is FDA-approved for use in conjunction with a reduced-calorie diet and increased physical activity. It is a powerful tool to aid these lifestyle changes, not replace them. Lasting success requires ongoing commitment to healthier habits.
Gradual Dosing: Treatment starts low (2.5 mg/week) and increases gradually every 4 weeks under medical guidance to improve tolerability of side effects, reaching a maintenance dose (typically 5 mg, 10 mg, or 15 mg).
- Important Safety Information & Side Effects (Critical Considerations):
“Saying goodbye” to excess weight shouldn’t mean ignoring potential health risks:
Common Side Effects: Primarily gastrointestinal – nausea, diarrhea, vomiting, constipation, decreased appetite, indigestion, abdominal pain. These are often dose-related and may decrease over time but can be significant for some users.
FDA Boxed Warning (Thyroid C-Cell Tumors): Tirzepatide has this warning based on rodent studies. It’s unknown if it causes these tumors in humans. It is contraindicated in patients with a personal or family history of Medullary Thyroid Carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome1 type 2 (MEN 2). Discuss any thyroid concerns with your doctor.
Other Serious Warnings: Pancreatitis (inflammation of the pancreas), gallbladder problems (gallstones), acute kidney injury (risk increased with dehydration from GI side effects), severe stomach problems, serious allergic reactions, hypoglycemia (especially if used with certain other diabetes medications), potential worsening of diabetic retinopathy in T2D patients.
Medical Supervision: Ongoing monitoring by your healthcare provider is essential to manage side effects, assess effectiveness, and ensure safety.
- Who is it For? (FDA Indications for Zepbound in the USA):
Zepbound is indicated for adults with:
Obesity (BMI ≥ 30 kg/m²) OR
Overweight (BMI ≥ 27 kg/m²) who also have at least one weight-related medical problem (such as high blood pressure, high cholesterol, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease).
- The Commitment: Long-Term Use & Access Realities in the USA:
Chronic Treatment: Obesity is a chronic disease. Clinical studies (like SURMOUNT-4) show that if Tirzepatide is stopped, weight regain is likely. Therefore, “saying goodbye” to excess weight generally implies committing to long-term, potentially lifelong, treatment to maintain the results.
Cost & Insurance: This is a major challenge in the US healthcare system.
Tirzepatide (Zepbound/Mounjaro) has a very high list price.
Insurance coverage varies drastically. Many employer plans, marketplace plans, and government plans (Medicare/Medicaid often have restrictions on anti-obesity medications) may not cover it, or may require significant prior authorizations and high co-pays.
Manufacturer savings cards can help offset costs for commercially insured patients, but often have limitations and don’t eliminate the underlying high price or lack of coverage.
The need for long-term treatment makes the financial aspect a critical factor for sustained use for many Americans.
Conclusion:
Tirzepatide (Zepbound) represents a significant leap forward in medical weight management, offering hope and unprecedented results for many individuals in the USA struggling with excess weight. Its powerful dual-hormone action targets the underlying biology of appetite and metabolism, making substantial weight loss achievable and giving rise to the feeling that one can finally “say goodbye” to the constant battle.
However, this powerful tool comes with important responsibilities and considerations. It requires a prescription and close medical supervision due to potential side effects and safety warnings. It necessitates a commitment to ongoing lifestyle changes (diet and exercise). Critically, achieving lasting results likely means committing to long-term treatment, which brings the significant hurdles of cost and insurance accessibility sharply into focus within the US system.
Therefore, while Tirzepatide offers a remarkable opportunity to manage excess weight effectively, “saying goodbye” involves embracing it as part of a comprehensive, long-term health strategy under professional guidance, with realistic expectations about commitment and access.
Say Goodbye to Excess Weight with Tirzepatide
Route
Doctor G Medical Excellence: Health Well-being and Longevity
URL: https://doctorgmed.com/
| Monday | 09:00 - 17:00 |
| Tuesday | 09:00 - 17:00 |
| Wednesday | 09:00 - 17:00 |
| Thursday | 09:00 - 17:00 |
| Friday | 09:00 - 17:00 |
| Saturday | 09:00 - 17:00 |
| Sunday | Closed |